Aminohippurate Sodium Injection
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
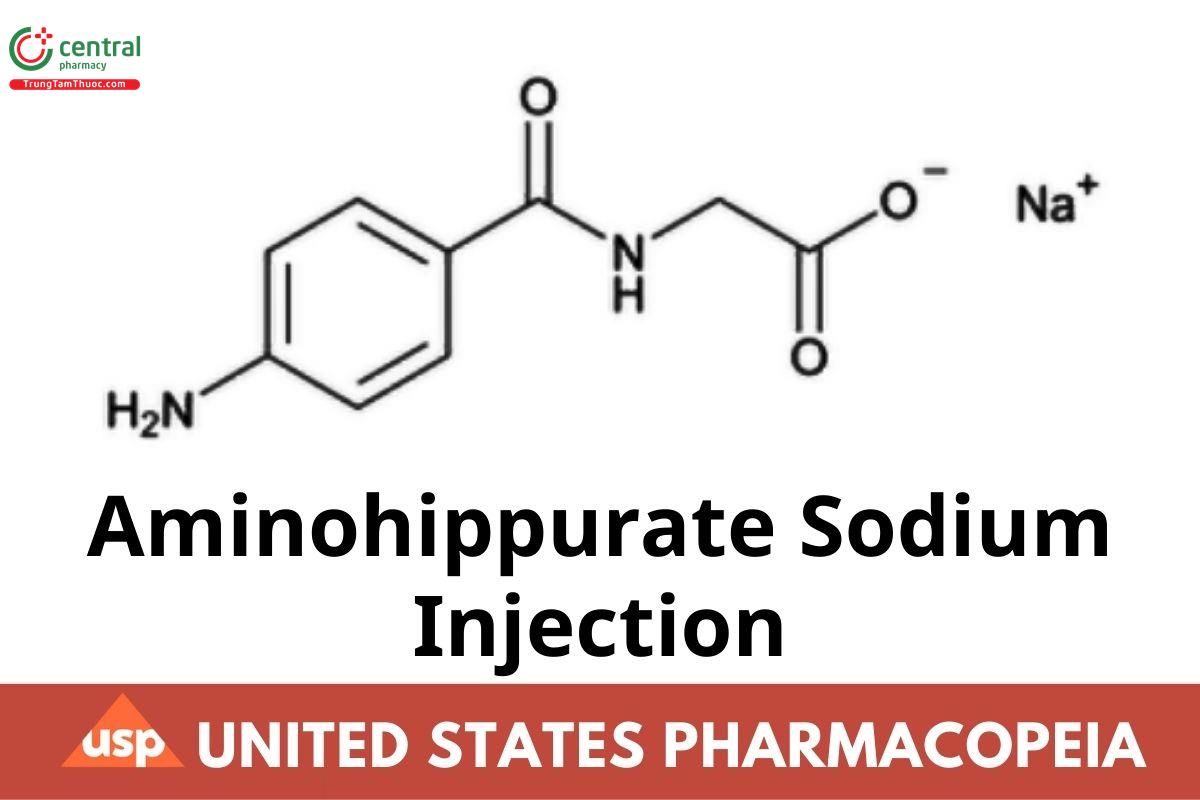
C9H9N2NaO3 216.17
Glycine, N-(4-aminobenzoyl)-, monosodium salt;
Monosodium p-aminohippurate CAS RN®: 94-16-6; UNII: SUO3KVS109.
1 DEFINITION
Aminohippurate Sodium Injection is a sterile solution of Aminohippuric Acid in Water for Injection prepared with the aid of Sodium Hydroxide. It contains NLT 95.0% and NMT 105.0% of the labeled amount of aminohippurate sodium (C9H9N2NaO3 ).
2 IDENTIFICATION
A.
Sample solution: A volume of Injection equivalent to 100 mg of aminohippuric acid
Analysis: Dilute the Sample solution with water to 50 mL. To 5 mL of this solution add 0.5 mL of 3 N hydrochloric acid, 0.5 mL of sodium nitrite solution (1 in 10), and a solution of 0.20 g of 2-naphthol in 10 mL of 6 N ammonium hydroxide.
Acceptance criteria: A red color is produced.
B.
Sample solution: A volume of Injection equivalent to about 200 mg of aminohippurate sodium
Analysis: In the order named, add to the Sample solution 2 mL of potassium iodide TS, 10 mL of water, and 5 mL of sodium hypochlorite TS.
Acceptance criteria: A red color is produced.
C. A sample imparts an intense yellow color to a nonluminous flame.
3 ASSAY
PROCEDURE
Sample solution: A volume of Injection equivalent to 1 g of aminohippurate sodium
Analysis: Transfer the Sample solution to a 200-mL volumetric flask, and dilute with water to volume. Transfer 50.0 mL of the solution to a suitable container, and add 5 mL of hydrochloric acid. Proceed as directed in Nitrite Titration (451), beginning with "cool to about 15°". Each mL of 0.1 M sodium nitrite is equivalent to 21.62 mg of aminohippurate sodium (C9H9N2NaO3 ).
Acceptance criteria: 95.0%-105.0%
4 SPECIFIC TESTS
pH (791): 6.7-7.6
BACTERIAL ENDOTOXINS TEST (85): NMT 0.04 USP Endotoxin Units/mg of aminohippurate sodium
OTHER REQUIREMENTS: Meets the requirements in Injections and Implanted Drug Products (1).
5 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in single-dose or multiple-dose containers, preferably of Type I glass.


