Aminobenzoate Potassium
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
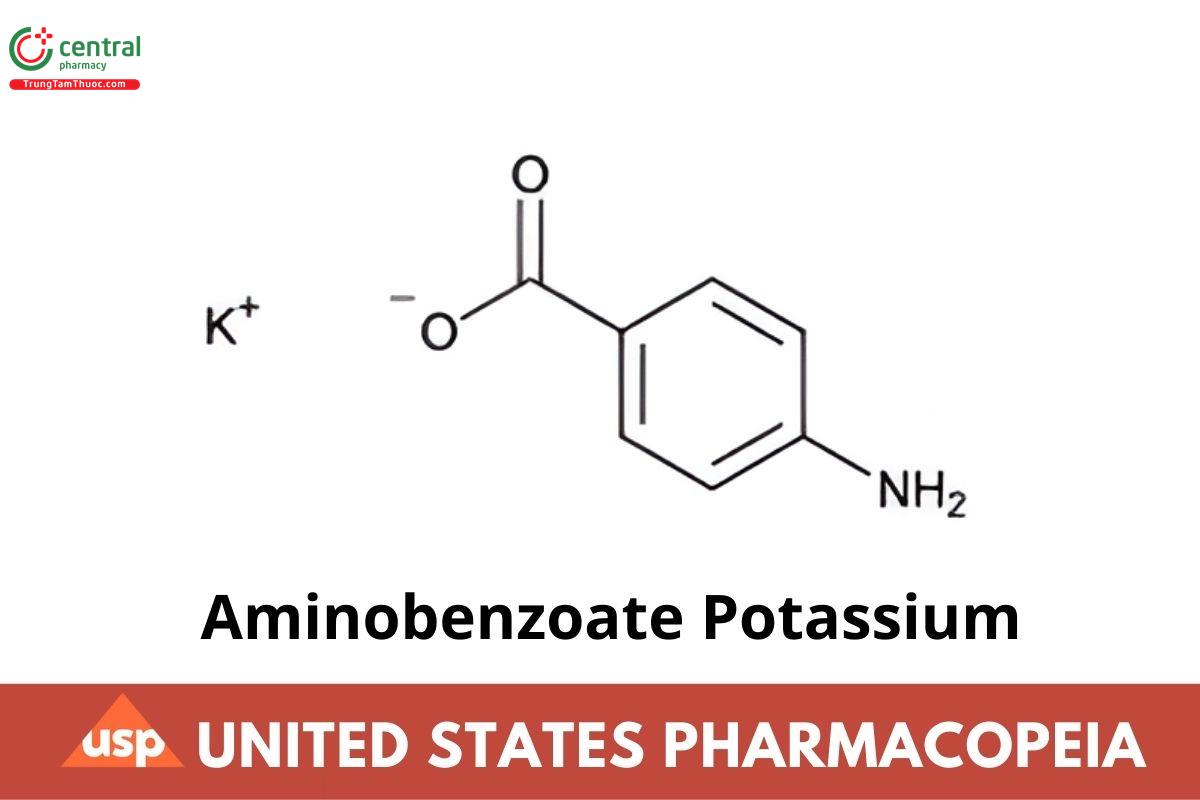
Aminobenzoate Potassium contains NLT 98.0% and NMT 102.0% of aminobenzoate potassium (C7H6KNO2), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
C.
Sample solution: 10 mg/mL of Aminobenzoate Potassium in water
Acceptance criteria: The Sample solution imparts a violet color to a nonluminous flame. Since the presence of small quantities of sodium masks the color, screen out the yellow color produced by sodium by viewing through a blue filter that blocks emission at 589 nm (sodium) but is transparent to emission at 404 nm (potassium). [Note—Traditionally, cobalt glass has been used, but other suitable filters are commercially available.]
3 ASSAY
3.1 Procedure
Solution A: 1.5% acetic acid, prepared by mixing 690 mL of water with 10 mL of glacial acetic acid and passing through a suitable filter of 0.45-μm pore size
Mobile phase: Methanol and Solution A (15:85)
Standard solution: 0.1 mg/mL of USP Aminobenzoate Potassium RS in Mobile phase. Sonicate to dissolve.
Sample solution: 0.1 mg/mL of Aminobenzoate Potassium in Mobile phase. Sonicate to dissolve.
3.2 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 3.0-mm × 15-cm; 3.5-μm packing L11
Flow rate: 0.35 mL/min
Injection volume: 5 μL
3.3 System suitability
Sample: Standard solution
3.4 Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 0.73%
3.5 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of aminobenzoate potassium (C7H6KNO2) in the portion of Aminobenzoate Potassium taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Aminobenzoate Potassium RS in the Standard solution (mg/mL)
Cu = concentration of Aminobenzoate Potassium in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the dried basis
4 IMPURITIES
Delete the following:
Heavy Metals 〈231〉, Method II: NMT 0.002% (Official 1-Jan-2018)
Change to read:
4.1 Limit of Aniline and p-Toluidine
Diluent: Methylene chloride
Standard stock solution: 0.1 mg/mL each of USP Aniline RS and USP p-Toluidine RS in Diluent
Standard solution: 1 μg/mL each of USP Aniline RS and USP p-Toluidine RS in Diluent from Standard stock solution
Sample solution: 100 mg/mL of Aminobenzoate Potassium in Diluent prepared as follows. Add an appropriate quantity of Aminobenzoate
Potassium to a suitable volumetric flask and dilute with Diluent to volume. Agitate for 10 min on a shaker and centrifuge at 3000 rpm for 5min. Use the supernatant.
4.1.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detectors
Flame ionization: 300°
Hydrogen: 40 mL/min
Air: 400 mL/min
Column: 30-m × 0.32-mm fused silica capillary; coated with 0.5-μm film of phase G27
Temperatures
Injection port: 280°
Detector: 300°
Column: See Table 1.
Table 1
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
|---|---|---|---|
| 130 | — | 130 | 4 |
| 130 | 20 | 180 | 5 |
Carrier gas: Helium
Flow rate: 1.0 mL/min
Injection volume: 2 μL
Injection type: Split ratio, 1:10
4.1.2 System suitability
Sample: Standard solution
[Note-The relative retention times of aniline and p-toluidine are 0.8 and 1.0, (ERR 1-Sep-2018) respectively.]
4.1.3 Suitability requirements
Resolution: NLT 7.0 between aniline and p-toluidine
Tailing factor: NMT 1.5 for aniline and p-toluidine
Relative standard deviation: NMT 6.0% for aniline and p-toluidine
4.1.4 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of p-toluidine or aniline in the portion of Aminobenzoate Potassium taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response of p-toluidine or aniline from the Sample solution
rs = peak response of p-toluidine or aniline from the Standard solution
Cs = concentration of the corresponding USP Reference Standard in the Standard solution (mg/mL)
Cu = concentration of Aminobenzoate Potassium in the Sample solution (mg/mL)
4.1.5 Acceptance criteria
Aniline: NMT 10 ppm
p-Toluidine: NMT 20 ppm
4.2 Organic Impurities
Solution A: 1.5% acetic acid, prepared by mixing 690 mL of water with 10 mL of glacial acetic acid
Solution B: Methanol
Mobile phase: See Table 2.
Table 2
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0.0 | 85 | 15 |
| 4.0 | 85 | 15 |
| 4.1 | 45 | 55 |
| 10.0 | 45 | 55 |
| 10.1 | 85 | 15 |
| 13 | 85 | 15 |
Diluent: Methanol and water (85:15)
System suitability solution: 1 mg/mL of USP Aminobenzoate Potassium RS, 0.01 mg/mL of USP 4-Nitrobenzoic Acid RS, and 0.01 mg/mL of USP Benzocaine RS in Diluent prepared as follows. Transfer 1 mL each of 0.1 mg/mL of USP 4-Nitrobenzoic Acid RS in methanol and 0.1 mg/mL of USP Benzocaine RS in Diluent to a 10-mL volumetric flask containing the appropriate amount of USP Aminobenzoate Potassium RS, and dilute with Diluent to volume.
Standard solution: 1 μg/mL each of USP Aminobenzoate Potassium RS, USP 4-Nitrobenzoic Acid RS, and USP Benzocaine RS in Diluent
Sample solution: 1 mg/mL of Aminobenzoate Potassium in Diluent
4.2.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 3.0-mm × 15-cm; 3.5-μm packing L11
Flow rate: 0.4 mL/min
Injection volume: 5 μL
4.2.2 System suitability
Samples: System suitability solution and Standard solution
4.2.3 Suitability requirements
Resolution: NLT 1.5 between benzocaine and 4-nitrobenzoic acid, System suitability solution
Relative standard deviation: NMT 3% for the aminobenzoate potassium, 4-nitrobenzoic acid, and benzocaine peaks, Standard solution
4.2.4 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of 4-nitrobenzoic acid or benzocaine in the portion of Aminobenzoate Potassium taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response of 4-nitrobenzoic acid or benzocaine from the Sample solution
rs = peak response of 4-nitrobenzoic acid or benzocaine from the Standard solution
Cs = concentration of USP 4-Nitrobenzoic Acid RS or USP Benzocaine RS in the Standard solution (mg/mL)
Cu = concentration of Aminobenzoate Potassium in the Sample solution (mg/mL)
Calculate the percentage of any individual unspecified impurity in the portion of Aminobenzoate Potassium taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response of any unspecified impurity from the Sample solution
rs = peak response of aminobenzoate from the Standard solution
Cs = concentration of USP Aminobenzoate Potassium RS in the Standard solution (mg/mL)
Cu = concentration of Aminobenzoate Potassium in the Sample solution (mg/mL)
Acceptance criteria: See Table 3.
Table 3
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
|---|---|---|
| Aminobenzoic acid | 1.0 | — |
| Benzocaine | 2.0 | 0.2 |
| 4-Nitrobenzoic acid | 2.1 | 0.2 |
| Any individual unspecified impurity | — | 0.10 |
5 SPECIFIC TESTS
pH 〈791〉
Sample solution: 50 mg/mL of Aminobenzoate Potassium in water
Acceptance criteria: 8.0–9.0
Loss on Drying 〈731〉
Analysis: Dry at 105° for 2 h.
Acceptance criteria: NMT 1.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers.
USP Reference Standards 〈11〉
USP Aminobenzoate Potassium RS
USP Aniline RS
Aniline.
C6H7N 93.13
USP Benzocaine RS
USP 4-Nitrobenzoic Acid RS
4-Nitrobenzoic acid.
C7 H5NO4 167.12
USP p-Toluidine RS
4-Methylaniline.
C7H9N 107.15


