Altretamine
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
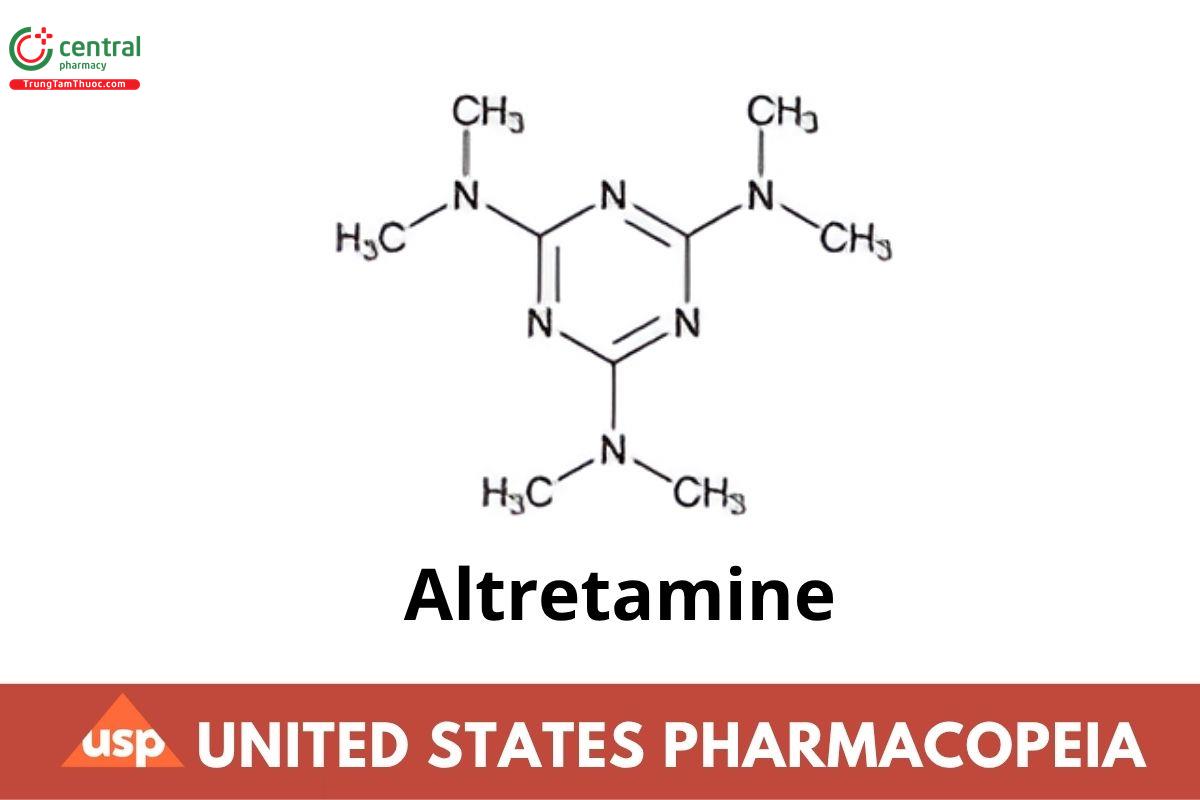
C9H18N6 210.28
1,3,5-Triazine-2,4,6-triamine, N,N,N′,N′,N′′,N′′-hexamethyl-;
Hexamethylmelamine CAS RN®: 645-05-6.
1 DEFINITION
Altretamine contains NLT 98.0% and NMT 102.0% of altretamine (C9H18N6), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy 197A or 197K (CN 1-May-2020) : Meets the requirements
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 Procedure
Solution A: 1.7 g/L of dibasic potassium phosphate in water. Adjust with phosphoric acid to a pH of 7.0 ± 0.1.
Solution B: Acetonitrile
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 3 | 100 | 0 |
| 25 | 50 | 50 |
| 28 | 50 | 50 |
| 29 | 100 | 0 |
| 35 | 100 | 0 |
Standard stock solution: 1.0 mg/mL of USP Altretamine RS prepared as follows. Dissolve a suitable amount of USP Altretamine RS in about 40% of the flask volume of acetonitrile. Sonicate as needed to dissolve. Add water almost to volume and allow the solution to reach room temperature. Dilute with water to volume and mix.
Standard solution: 0.1 mg/mL of USP Altretamine RS in water from Standard stock solution
Sample stock solution: 1.0 mg/mL of Altretamine prepared as follows. Dissolve a suitable amount of Altretamine in about 40% of the flask volume of acetonitrile. Sonicate as needed to dissolve. Add water almost to volume and allow the solution to reach room temperature.
Dilute with water to volume and mix.
Sample solution: 0.1 mg/mL of Altretamine in water from Sample stock solution
3.2 Chromatographic system
Mode: LC
Detector: UV 242 nm
Column: 4.6-mm × 25-cm; 5-μm packing L96
Flow rate: 1.5 mL/min
Injection volume: 20 μL. [Note - An in-line filter may be needed to reduce system pressure.]
3.3 System suitability
Sample: Standard solution
3.4 Suitability requirements
Tailing factor: NMT 1.5
Relative standard deviation: NMT 2.0%
3.5 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of altretamine (C9H18N6) in the portion of Altretamine taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Altretamine RS in the Standard solution (mg/mL)
Cu = concentration of Altretamine in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Organic Impurities
Solution A, Solution B, Mobile phase, Standard solution, and Sample solution: Prepare as directed in the Assay.
Sensitivity solution: 0.06 μg/mL of USP Altretamine RS in water from Standard solution
Chromatographic system
Mode: LC
Detector: UV 215 and 242 nm (see Table 2)
Column: 4.6-mm × 25-cm; 5-μm packing L96
Flow rate: 1.5 mL/min
Injection volume: 20 μL
System suitability
Samples: Standard solution and Sensitivity solution
Suitability requirements
Tailing factor: NMT 1.5, Standard solution
Relative standard deviation: NMT 2.0%, Standard solution
Signal-to-noise ratio: NLT 10 for the altretamine peak, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Altretamine taken:
Result = (ru/rs) × (Cs/Cu) × (1/F) × 100
ru = peak response of each impurity from the Sample solution
rs = peak response of altretamine from the Standard solution
Cs = concentration of USP Altretamine RS in the Standard solution (mg/mL)
Cu = concentration of Altretamine in the Sample solution (mg/mL)
F = relative response factor (see Table 2)
Acceptance criteria: See Table 2. The reporting threshold is NMT 0.06%.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Detector (nm) | Acceptance Criteria, NMT (%) |
|---|---|---|---|---|
| Altretamine diketo analoga | 0.21 | 0.44 | 242 | 0.1 |
| Altretamine chloro keto analogb | 0.35 | 0.60 | 215 | 0.1 |
| Altretamine keto analogc | 0.56 | 1.3 | 242 | 0.1 |
| Altretamine dichloro analogd | 0.87 | 0.87 | 242 | 0.1 |
| Altretamine monochloro analoge | 0.96 | 1.3 | 242 | 0.1 |
| Altretamine | 1.00 | 1.0 | 215, 242f | — |
| Any other individual impurities | — | — | 242 | 0.1 |
| Total impurities | — | — | — | 0.3 |
a 6-(Dimethylamino)-1,3,5-triazine-2,4[1H,3H]-dione.
b 4-Chloro-6-(dimethylamino)-1,3,5-triazine-2[1H]-one.
c 4,6-Bis(dimethylamino)-1,3,5-triazine-2[1H]-one.
d 4,6-Dichloro-N,N-dimethyl-1,3,5-triazine-2-amine.
e 6-Chloro-,N2,N2,N4,N4 - tetramethyl-1,3,5-triazine-2,4-diamine.
f Use the detector consistent with respective impurity.
5 SPECIFIC TESTS
Water Determination 〈921〉, Method I: NMT 1%
Tests for Specified Microorganisms 〈62〉: The total aerobic microbial count is NMT 103 cfu/g. The total combined molds and yeasts count is NMT 102cfu/g.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers, and store at controlled room temperature.
USP Reference Standards 〈11〉
USP Altretamine RS


