Alprostadil
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
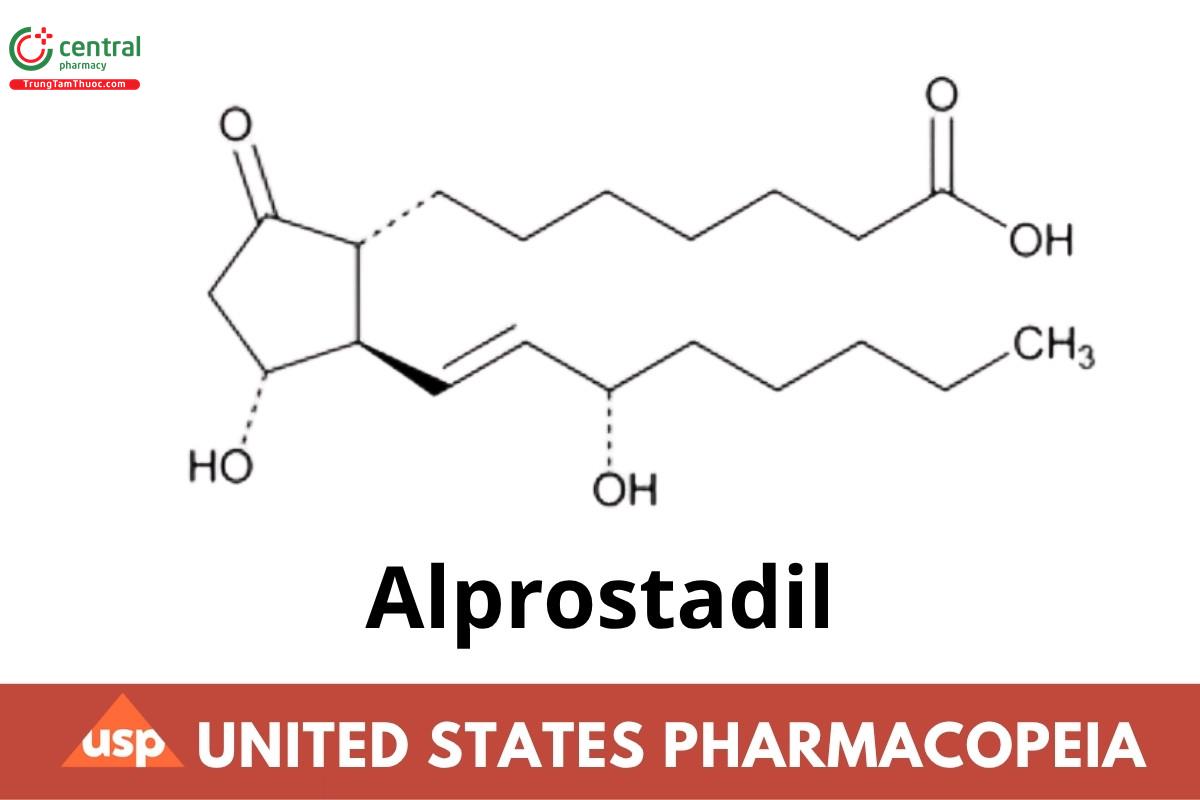
C20H34O5 354.48
Prost-13-en-1-oic acid, 11,15-dihydroxy-9-oxo-, (11α, 13Ε,155)-;
(1R,2R,3R)-3-Hydroxy-2-[(E)-(3S)-3-hydroxy-1-octenyl]-5-oxocyclopentaneheptanoic acid CAS RN: 745-65-3; UNII: F5TD010360.
1 DEFINITION
Alprostadil contains NLT 95.0% and NMT 105.0% of alprostadil (C20H340g), calculated on the anhydrous basis.
[CAUTION-Great care should be taken to prevent inhaling particles of Alprostadil and exposing the skin to it.]
2 IDENTIFICATION
Change to read:
A. (USP 1-MAY-2022) SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197M
3 ASSAY
3.1 PROCEDURE
Use freshly prepared solutions.
Mobile phase: Methanol, acetonitrile, and 0.1 M monobasic potassium phosphate (2:1:2). Adjust with phosphoric acid to a pH of 3.0.
Diluent: Methanol and water (90:10)
Internal standard solution: 0.05 mg/mL of ethylparaben in Diluent
Standard stock solution: 0.3 mg/mL of USP Alprostadil RS in Diluent
Standard solution: 0.2 mg/mL of USP Alprostadil RS prepared by combining 2.0 mL of Standard stock solution with 1.0 mL of Internal standard solution
System suitability stock solution: 4.5 µg/mL of USP Prostaglandin A1 RS in Standard stock solution
System suitability solution: Combine 2.0 mL of System suitability stock solution with 1.0 mL of Internal standard solution.
Sample stock solution: 0.3 mg/mL of Alprostadil in Diluent
Sample solution: 0.2 mg/mL of Alprostadil prepared by combining 2.0 mL of Sample stock solution and 1.0 mL of Internal standard solution
3.2 Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: Photodiode array detector or equivalent capable of detecting UV wavelengths of 200-300 nm
Analytical wavelength: 200 nm
Column: 4.6-mm x 25-cm; packing L1
Column temperature: 40°
Flow rate: 1 mL/min
Injection volume: 20 µL
System suitability
Sample: System suitability solution
Suitability requirements
Resolution: NLT 7.5 between prostaglandin A1 and alprostadil, and NLT 2.0 between prostaglandin A1 and ethylparaben
Relative standard deviation: NMT 2.0%, determined from the peak area ratio of alprostadil to ethylparaben
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of alprostadil (C20H34O5) in the portion of Alprostadil taken:
Result = (RU/RS) x (CS/CU) x 100
RU = peak area ratio of alprostadil to the internal standard from the Sample solution
RS = peak area ratio of alprostadil to the internal standard from the Standard solution
CS = concentration of USP Alprostadil RS in the Standard solution (mg/mL)
CU = concentration of Alprostadil in the Sample solution (mg/mL)
Acceptance criteria: 95.0%-105.0% on the anhydrous basis
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281)
Sample: 0.3 g
Acceptance criteria: NMT 0.5%
Delete the following:
4.2 LIMIT OF CHROMIUM
Standard stock solution: 3.04 µg/mL of chromium trichloride in 0.05 M nitric acid
Standard solution: 20 ng/mL of chromium (Cr) in alcohol, prepared as follows. Transfer 2 mL of the Standard stock solution to a 100-mL volumetric flask, and dilute with alcohol to volume.
Sample solution: 1.0 mg/mL of Alprostadil in alcohol
Blank: Alcohol
Instrumental conditions
(See Atomic Absorption Spectroscopy (852).)
Mode: Atomic absorption spectroscopy
Lamp: Chromium hollow-cathode
Analytical wavelength: 357.9 nm
Atomization type: Graphite furnace
Temperatures
Drying: 100°
Ashing: 1000°
Atomization: 2700°
Injection volume: 20 µL
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of chromium (Cr) in the portion of Alprostadil taken:
Result = (AU/AS) × (CS/CU) × 100
AU = absorbance of the Sample solution
AS = absorbance of the Standard solution
CS = concentration of chromium in the Standard solution (mg/mL)
CU = concentration of Alprostadil in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.002% (USP 1-May-2022)
Delete the following:
4.3 LIMIT OF RHODIUM
Standard stock solution: 100 µg/mL of rhodium in 1.2 M hydrochloric acid, prepared by diluting rhodium chloride hydrate
Standard solution: 50 ng/mL of rhodium (Rh) in alcohol from Standard stock solution
Sample solution: 2.0 mg/mL of Alprostadil in alcohol
Blank: Alcohol
Instrumental conditions
(See Atomic Absorption Spectroscopy (852).)
Mode: Atomic absorption spectroscopy
Lamp: Rhodium hollow-cathode
Analytical wavelength: 343.5 nm
Atomization type: Graphite furnace
Temperatures
Drying: 100°
Ashing: 1000°
Atomization: 2800°
Injection volume: 20 µL
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of rhodium (Rh) in the portion of Alprostadil taken:
Result = (AU/AS) × (CS/CU) × 100
AU = absorbance of the Sample solution
AS = absorbance of the Standard solution
CS = concentration of rhodium in the Standard solution (mg/mL)
CU = concentration of Alprostadil in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.002% (USP 1-May-2022)
Change to read:
4.4 LIMIT OF FOREIGN PROSTAGLANDINS, TEST 1
Use freshly prepared solutions.
Mobile phase: Methanol, acetonitrile, and 0.1 M monobasic potassium phosphate (2:1:2). Adjust with phosphoric acid to a pH of 3.0.
Diluent: Methanol and water (90:10)
Standard solution: 6 µg/mL of USP Alprostadil RS, 15 µg/mL of USP Prostaglandin A1 RS, and 6 µg/mL of USP Prostaglandin B1 RS in Diluent
Sample solution: 3.0 mg/mL of Alprostadil in Diluent
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: Photodiode array detector or equivalent capable of detecting UV wavelengths of 200-300 nm
Analytical wavelengths
Prostaglandin A1: 224 nm
Prostaglandin B1: 280 nm
Other foreign prostaglandins: 200 nm
Column: 4.6-mm x 25-cm; packing L1
Column temperature: 40°
Flow rate: 1 mL/min
Injection volume: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 7.5 between prostaglandin A, and alprostadil
Relative standard deviation: NMT 4.0%, determined from the peaks at their respective wavelength for replicate injections
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of prostaglandin A1 and prostaglandin B1 in the portion of Alprostadil taken:
Result = (rU/rS) x (CS/CU) x 100
rU = peak response of prostaglandin A1 or prostaglandin B1 from the Sample solution
rS = peak response of prostaglandin A1 or prostaglandin B1 from the Standard solution
CS = concentration of USP Prostaglandin A1 RS or USP Prostaglandin B1 RS in the Standard solution (mg/mL)
CU = concentration of Alprostadil in the Sample solution (mg/mL)
Calculate the percentage of each impurity occurring at 200 nm and eluting before prostaglandin A, in the portion of Alprostadil taken:
Result = (rU/rS) x (CS/CU) x 100
rU = peak response for each impurity from the Sample solution
rS = peak response for alprostadil from the Standard solution
CS = concentration of USP Alprostadil RS in the Standard solution (mg/mL)
CU = concentration of Alprostadil in the Sample solution (mg/mL)
Calculate the percentage of any (USP 1-May-2022) impurity having a relative retention time of 0.6, relative to the prostaglandin A1 peak detected at 224 nm, in the portion of Alprostadil taken:
Result = (rU/rS) x (CS/CU) x 100
rU = peak response for any impurity having a relative retention time of 0.6, relative to the prostaglandin A, peak, from the Sample solution
rS = peak response for prostaglandin A, from the Standard solution
CS = concentration of USP Prostaglandin A1 RS in the Standard solution (mg/mL)
CU = concentration of Alprostadil in the Sample solution (mg/mL)
Acceptance criteria
Prostaglandin A1: NMT 1.5%
Prostaglandin B1: NMT 0.1%
Any foreign prostaglandin impurity eluting before prostaglandin A1: NMT 0.9%
Impurity at relative retention time 0.6, relative to prostaglandin A1: NMT 0.9%
Change to read:
4.5 LIMIT OF FOREIGN PROSTAGLANDINS, TEST 2
Mobile phase: Methanol, acetonitrile, and 0.02 M monobasic potassium phosphate (2:1:1). Adjust with phosphoric acid to a pH of 3.
System suitability solution: 6 µg/mL of USP Alprostadil RS, 15 µg/mL of USP Prostaglandin A1 RS, and 6 µg/mL of USP Prostaglandin B1 RS in methanol and water (9:1)
Standard solution: 10 µg/mL of USP Alprostadil RS in acetonitrile and water (1:1)
Sample solution: 5.0 mg/mL of Alprostadil in acetonitrile and water (1:1). [NOTE-Sonicate if necessary.] necessary.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: Photodiode array detector or equivalent, capable of detecting UV wavelengths of 200-300 nm FICIAL
Analytical wavelengths
Prostaglandin A1: 224 nm
Prostaglandin B1: 280 nm
Other foreign prostaglandins: 200 nm
Column: 4.6-mm x 25-cm; packing L1
Flow rate: 1.2 mL/min
Injection volume: 20 µL
System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times for alprostadil and prostaglandin A, are 1.0 and 1.2, respectively. (USP 1-May-2022)]
Suitability requirements
Resolution: NLT 4.0 between prostaglandin A, and alprostadil, System suitability solution
Relative standard deviation: NMT 2.0%, determined from the main peak, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity, excluding prostaglandin B,, observed at 200 nm and eluting after prostaglandin A1 in the portion of Alprostadil taken:
Result = (rU/rS) x (CS/CU) x 100
rU = peak response for each impurity from the Sample solution
rS = peak response for alprostadil from the Standard solution
CS = concentration of USP Alprostadil RS in the Standard solution (mg/mL)
CU = concentration of Alprostadil in the Sample solution (mg/mL)
Acceptance criteria
Sum of the impurities at relative retention times 2.0 and 2.3: NMT 0.6%
Any other foreign prostaglandin impurity eluting after prostaglandin A1: NMT 0.9%
Total impurities: The sum of the impurities from Limit of Foreign Prostaglandins, Test 1 and Test 2, is NMT 2.0%.
5 SPECIFIC TESTS
WATER DETERMINATION (921), Method I
Sample: 0.5 g
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers, and store in a refrigerator.
USP REFERENCE STANDARDS (11).
USP Alprostadil RS
USP Prostaglandin A1 RS
USP Prostaglandin B1 RS
(13E,15S)-15-Hydroxy-9-oxoprosta-8(12), 13-dien-1-oic acid.
C20H32O4 336.47


