Allantoin
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
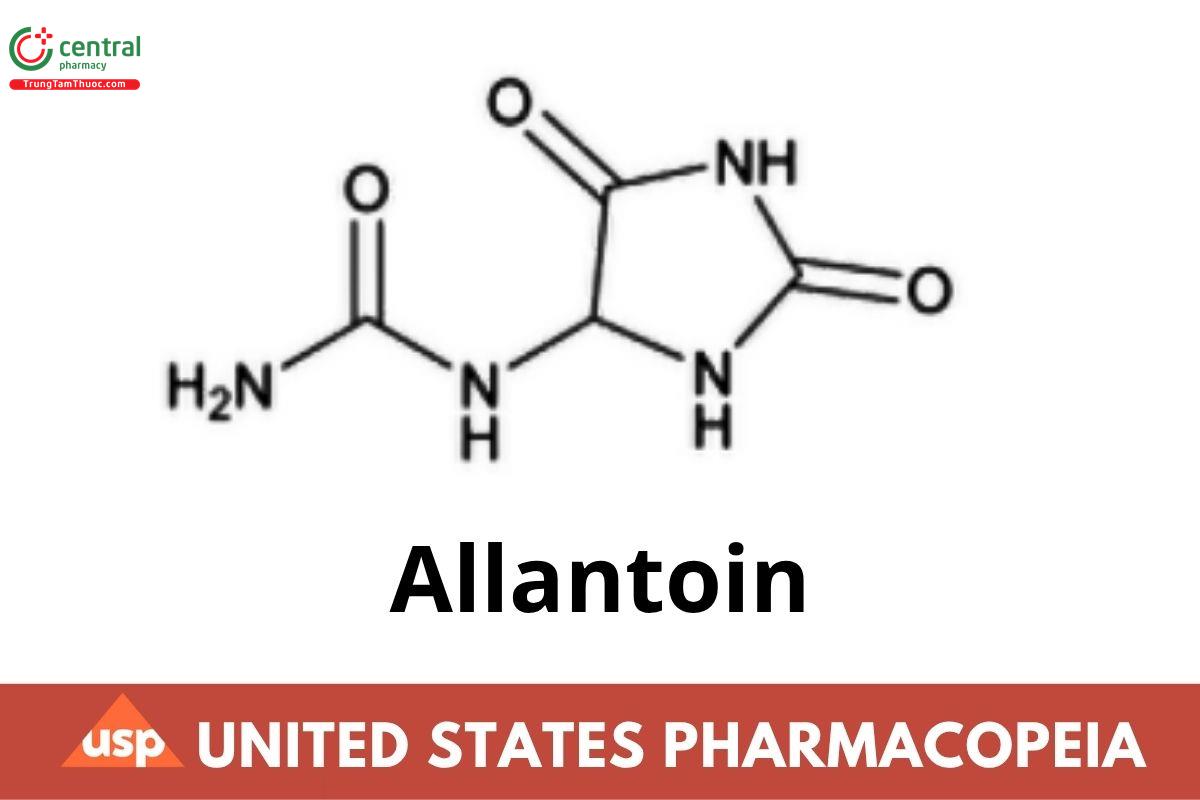
C4H6N4O3 158.12
Urea, (2,5-dioxo-4-imidazolidinyl)-;
Allantoin CAS RN®: 97-59-6; UNII: 344S277G0Z.
1 DEFINITION
Allantoin contains NLT 98.5% and NMT 101.0% of C4H6N4O3.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B. Thin-Layer Chromatographic Identification Test 〈201〉: The RF value of the principal spot from Sample solution B corresponds to that from Standard solution A, as described in the test for Organic Impurities.
3 ASSAY
Procedure
Sample: 120 mg
Analysis: Transfer the Sample to a 100-mL beaker, dissolve by stirring in 40 mL of water, and titrate with 0.1 M sodium hydroxide. Use a suitable electrode system (see Titrimetry 〈541〉). Each mL of 0.1 M sodium hydroxide is equivalent to 15.81 mg of C4H6N4O3.
Acceptance criteria: 98.5%–101.0%
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Organic Impurities
Adsorbent: Cellulose
Diluent: Methanol and water (1:1)
Urea stock solution: 1 mg/mL of USP Urea RS in water
Standard solution A: 1 mg/mL of USP Allantoin RS in Diluent
Standard solution B: 0.1 mg/mL of USP Urea RS in methanol, from Urea stock solution
Standard solution C: Standard solution A and Standard solution B (1:1)
Sample solution A: Transfer 0.10 g of Allantoin to a 10-mL volumetric ask, add 5 mL of water, dissolve by heating, and allow to cool. Dilute with methanol to volume. [Note—Use immediately after preparation.]
Sample solution B: Transfer 1 mL of Sample solution A to a 10-mL volumetric ask, and dilute with Diluent to volume. Spray reagent: 5 mg/mL of p-dimethylaminobenzaldehyde in a mixture of methanol and hydrochloric acid (3:1)
Application volume
Standard solution A: 5 µL
Standard solution B: 5 µL
Standard solution C: 5 µL
Sample solution A: 10 µL
Sample solution B: 5 µL
Developing solvent system: Butyl alcohol, glacial acetic acid, and water (60:15:25)
Analysis: Proceed as directed for Chromatography 〈621〉, Thin-Layer Chromatography. Develop the chromatogram until the solvent front has moved about 10 cm. Spray the plate with Spray reagent, dry in a current of hot air, and after 30 min examine under visible light. Acceptance criteria: Any spot from Sample solution A, except for the principal spot, is not more intense than the spot from Standard solution B (NMT 0.5%). The test is not valid unless the principal spots from Standard solution C are clearly separated.
5 SPECIFIC TESTS
5.1 Acidity or Alkalinity
Sample solution: 5 mg/mL in carbon dioxide-free water
Analysis: To 5 mL of the Sample solution add 5 mL of water, 0.1 mL of methyl red TS, and 0.2 mL of 0.01 M sodium hydroxide. Acceptance criteria: A yellow color is observed. The solution turns red upon the addition of 0.4 mL of 0.01 M hydrochloric acid.
Loss on Drying 〈731〉: Dry a sample at 105° to constant weight: it loses NMT 0.1% of its weight.
5.2 Reducing Substances
Sample solution: 1.0 g of Allantoin in 10 mL of water. Shake for 2 min, and filter.
Analysis: To the Sample solution add 1.5 mL of 0.02 M potassium permanganate.
Acceptance criteria: The solution remains violet for at least 10 min.
6 ADDITIONAL REQUIREMENTS
USP Reference Standards 〈11〉
USP Allantoin RS
USP Urea RS


