Alfuzosin Hydrochloride Extended-Release Tablets
If you find any inaccurate information, please let us know by providing your feedback here

Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Alfuzosin Hydrochloride Extended-Release Tablets contain NLT 90.0% and NMT 110.0% of the labeled amount of alfuzosin hydrochloride (C19H27N5O4 · HCl).
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A or 197K.
Sample: Grind 4 Tablets, and add 20 mL of water. [Note—When analyzing multi-layer Tablets, isolate the layer containing alfuzosin hydrochloride using a suitable tool.] Add 20 mL of strong ammonia solution. Extract with 20 mL of methylene chloride, and separate the organic layer. Repeat the extraction successively with 20 mL, then with 10 mL of methylene chloride. Wash the combined organic layers with 20 mL of water. Dry the organic solution using a phase separation lter. Take 2.0 mL of the dried organic solution, and mix with 200 mg of nely ground potassium bromide. Evaporate the methylene chloride at 60°, then at 105° for 30 min. Make a disk. Alternatively, evaporate methylene chloride from the dried organic solution at 60°, then at 105° for 30 min. Perform the IR spectrum.
Acceptance criteria: The maxima of the spectrum obtained from the Sample correspond in position and relative intensity to those obtained from USP Alfuzosin Hydrochloride RS, treated in the same manner as the Sample, beginning with “add 20 mL of water.” • B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 Procedure
Solution A: 5.0 mL of perchloric acid in 900 mL of water. Adjust with 2 M sodium hydroxide to a pH of 3.5, and dilute with water to 1000 mL. Mobile phase: Acetonitrile, tetrahydrofuran, and Solution A (20:1:80)
Diluent: 0.01 N hydrochloric acid
Standard stock solution: 0.15 mg/mL of USP Alfuzosin Hydrochloride RS in methanol
Standard solution: 0.03 mg/mL of USP Alfuzosin Hydrochloride RS in Diluent from the Standard stock solution
Sample stock solution: Place a suitable number of Tablets into a suitable volumetric ask to obtain a solution having a concentration of 0.16 mg/mL of alfuzosin hydrochloride. Add 80% of the ask volume of methanol, and stir for at least 1 h using a magnetic stirrer. Add 10% of the ask volume of Diluent, mix, and allow it to cool to room temperature. Dilute the resulting suspension with methanol to volume, stir, and allow to settle for 30 min.
Sample solution: 0.03 mg/mL of alfuzosin hydrochloride in Diluent from the Sample stock solution supernatant. Pass through a suitable lter. Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 15-cm; 5-µm packing L1
Flow rate: 1.5 mL/min
Injection volume: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: 0.8–1.5 for alfuzosin
Relative standard deviation: NMT 2.0%
3.2 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of alfuzosin hydrochloride (C19H27N5O4 · HCl) in the portion of Tablets taken:
Result = (rU /rS ) × (CS /CU ) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Alfuzosin Hydrochloride RS in the Standard solution (mg/mL)
CU = nominal concentration of the Sample solution (mg/mL)
Acceptance criteria: 90.0%–110.0%
4 PERFORMANCE TESTS
4.1 Dissolution 〈711〉
Test 1
Medium: 0.01 N hydrochloric acid; 500 mL
Apparatus 2: 100 rpm, with Tablet holder (see Figure 1)
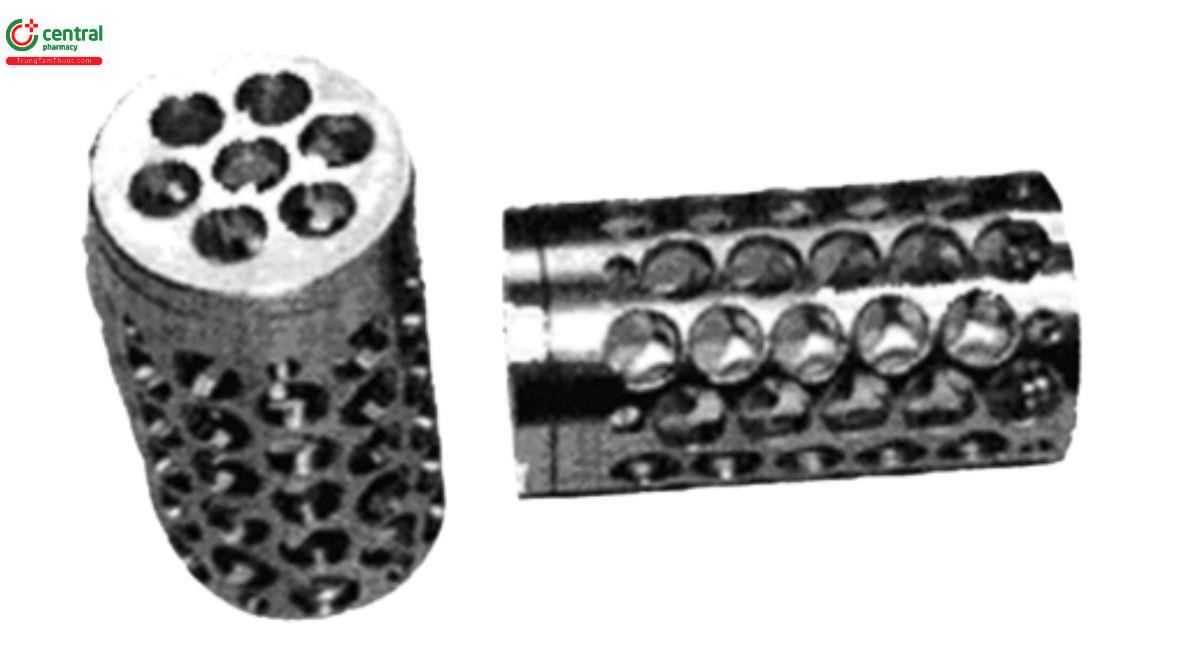
Times: 1, 6, 12, and 20 h
Sample solution: Pass a portion of the solution under test through a suitable lter.
Standard solution: (L/500) mg/mL of USP Alfuzosin Hydrochloride RS in Medium, where L is the Tablet label claim in mg Detector: UV 330 nm
Blank: Medium
Path length: 1 cm
Tolerances: See Table 1.
Table 1
Level | Time (h) | Amount Dissolved (%) |
L1 | Each Tablet: | |
1 | 10–20 | |
6 | 40–55 | |
12 | 65–85 | |
20 | NLT 85 | |
L2 | Average of 12 Tablets complies with L1 and each Tablet: | |
1 | 9–22 | |
6 | 36–61 | |
12 | 59–94 | |
20 | NLT 77 | |
L3 |
| Average of 24 Tablets complies with L1, NMT 2 Tablets outside L2, and all Tablets within: |
1 | 8–24 | |
6 | 32–66 | |
12 | 52–102 | |
20 | NLT 68 |
Test 2: If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 2.
Medium: 0.01 N hydrochloric acid; 900 mL
Apparatus 2: 100 rpm
Times: 1, 3, 12, and 24 h
Buffer: Dilute 5.0 mL of perchloric acid in 900 mL of water, adjust with diluted sodium hydroxide (0.1 g/mL) to a pH of 3.5 ± 0.5, and dilute with water to 1 L.
Mobile phase: Acetonitrile and Buffer (25:75)
Standard stock solution: 0.28 mg/mL of USP Alfuzosin Hydrochloride RS, prepared as follows. In a 200-mL volumetric ask dissolve 55.5 mg of USP Alfuzosin Hydrochloride RS in 5 mL of methanol, sonicate to dissolve, and dilute with Medium to volume. Standard solution: 0.011 mg/mL of USP Alfuzosin Hydrochloride RS in Medium from the Standard stock solution Sample solution: Pass a portion of the solution under test through a suitable lter. Replace the portion of solution withdrawn with an equal volume of Medium.
4.2 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 244 nm
Column: 4.6-mm × 15-cm; 5-µm packing L7
Column temperature: 30°
Flow rate: 1.2 mL/min
Injection volume: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Column eciency: NLT 3000 theoretical plates
Relative standard deviation: NMT 2.0%
4.3 Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (C ) of alfuzosin hydrochloride (C19H27N5O4 · HCl) in the sample withdrawn from the vessel at each time point (i):
Resulti = (rU /rS ) × CS
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Alfuzosin Hydrochloride RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of alfuzosin hydrochloride (C19H27N5O4 · HCl) dissolved at each time point (i):
Result1 = C1 × V × (1/L) × 100
Result2 = [(C2 × V) + (C1 × VS )] × (1/L) × 100
Result3 = {(C3 × V) + [(C2 + C1 ) × VS ]} × (1/L) × 100
Result4 = {(C4 × V) + [(C3 + C2 + C1 ) × VS ]} × (1/L) × 100
Ci = concentration of alfuzosin hydrochloride in the portion of sample withdrawn at the specied time point (mg/mL)
V = volume of Medium, 900 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn at each time point and replaced with Medium (mL)
Tolerances: See Table 2.
Time Point (i) | Time (h) | Amount Dissolved (%) |
1 | 1 | NMT 20 |
2 | 3 | 15–35 |
3 | 12 | 50–70 |
4 | 24 | NLT 80 |
The percentages of the labeled amount of alfuzosin hydrochloride dissolved at the times specied conform to Dissolution 〈711〉, Acceptance Table 2.
Test 3: If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 3.
Medium: 0.25% sodium dodecyl sulfate in 0.05 M sodium phosphate buffer, pH 6.8 (2.5 g/L of sodium dodecyl sulfate, 6.9 g/L of monobasic sodium phosphate monohydrate, and 0.83 g/L of sodium hydroxide in water previously degassed with helium. Adjust with either phosphoric acid or 1 N sodium hydroxide to a pH of 6.8 ± 0.05); 900 mL
Apparatus 1: 100 rpm
Times: 1, 6, 12, and 24 h
Standard stock solution: 1.1 mg/mL of USP Alfuzosin Hydrochloride RS in methanol
Standard solution: 0.011 mg/mL of USP Alfuzosin Hydrochloride RS in Medium from the Standard stock solution
Sample solution: Pass a portion of the solution under test through a suitable lter.
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy 〈857〉.)
Mode: UV-Vis
Analytical wavelength: 331 nm, background correction at 490 nm
Blank: Medium
Cell: 1.0 cm
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of alfuzosin hydrochloride (C19H27N5O4 · HCl) dissolved at each time point (i):
Resulti = (AU /AS ) × CS × V × (1/L) × 100
AU = absorbance of the Sample solution
AS = absorbance of the Standard solution
CS = concentration of USP Alfuzosin Hydrochloride RS in the Standard solution (mg/mL)
V = volume of Medium (mL)
L = label claim (mg/Tablet)
Tolerances: See Table 3.
Table 3
Time Point (i) | Time (h) | Amount Dissolved (%) |
1 | 1 | NMT 15 |
2 | 6 | 20–40 |
3 | 12 | 45–70 |
4 | 24 | NLT 80 |
The percentages of the labeled amount of alfuzosin hydrochloride dissolved at the times specied conform to Dissolution 〈711〉, Acceptance Table 2.
Test 4: If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 4.
Medium: 0.01 N hydrochloric acid; 900 mL
Apparatus 2: 100 rpm
Times: 1, 6, 12, and 20 h
Standard solution: 0.01 mg/mL of USP Alfuzosin Hydrochloride RS in Medium
Sample solution: Centrifuge a portion of the solution under test.
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy 〈857〉.)
Mode: UV
Analytical wavelength: 245 nm
Blank: Medium
Path length: 0.2 cm Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (Ci) of alfuzosin hydrochloride (C19H27N5O4 · HCl) in the sample withdrawn from the vessel at each time
point (i):
Resulti = (AU/AS ) × CS
AU = absorbance of the Sample solution
AS = absorbance of the Standard solution
CS = concentration of USP Alfuzosin Hydrochloride RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of alfuzosin hydrochloride (C19H27N5O4 · HCl) dissolved at each time point (i):
Result1 = C1 × V × (1/L) × 100
Result2 = [(C2 × V) + (C1 × VS )] × (1/L) × 100
Result3 = {(C3 × V) + [(C2 + C1 ) × VS ]} × (1/L) × 100
Result4 = {(C4 × V) + [(C3 + C2 + C1 ) × VS ]} × (1/L) × 100
Ci = concentration of alfuzosin hydrochloride in the portion of sample withdrawn at the specied time point (mg/mL) i
V = volume of Medium, 900 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn at each time point (mL)
Tolerances: See Table 4.
Table 4
Time Point (i) | Time (h) | Amount Dissolved (%) |
1 | 1 | NMT 30 |
2 | 6 | 40–60 |
3 | 12 | 65–85 |
4 | 20 | NLT 80 |
The percentages of the labeled amount of alfuzosin hydrochloride dissolved at the times specied conform to Dissolution 〈711〉, Acceptance Table 2.
Test 5: If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 5.
Medium: 0.01 N hydrochloric acid; 900 mL
Apparatus 2: 100 rpm
Times: 1, 3, 6, 12, and 20 h
Buffer: Add 1 mL of triethylamine in 1000 mL of water. Adjust with phosphoric acid to a pH of 2.5 ± 0.05. Pass through a suitable lter of 0.45-µm pore size.
Mobile phase: Methanol and Buffer (40:60)
Standard stock solution: 0.55 mg/mL of USP Alfuzosin Hydrochloride RS. Prepare by transferring a portion of USP Alfuzosin Hydrochloride RS to a suitable ask. Add methanol to 20% of the ask volume, and sonicate at room temperature to dissolve. Dilute with Medium to volume Standard solution: 0.011 mg/mL of USP Alfuzosin Hydrochloride RS in Medium from the Standard stock solution. Pass through a suitable lter of 0.45-µm pore size.
Sample solution: Pass a portion of the solution under test through a suitable lter of 0.45-µm pore size.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 245 nm
Column: 4.6-mm × 15-cm; 5-µm packing L1
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Column eciency: NLT 2000 theoretical plates
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (Ci) of alfuzosin hydrochloride (C19H27N5O4· HCl) in the sample withdrawn from the vessel at each time point (i):
Resulti = (rU /rS ) × CS
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Alfuzosin Hydrochloride RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of alfuzosin hydrochloride (C19H27N5O4 · HCl) dissolved at each time point (i):
Result1 = C1 × V × (1/L) × 100
Result2 = [(C2 × V) + (C1 × VS )] × (1/L) × 100
Result3 = {(C3 × V) + [(C2 + C1 ) × VS ]} × (1/L) × 100
Result4 = {(C4 × V) + [(C3 + C2 + C1 ) × VS ]} × (1/L) × 100
Result5 = ({C5 × [V − (4 × VS )]} + [(C4 + C3 + C2 + C1 ) × VS]) × (1/L) × 100
Ci = concentration of alfuzosin hydrochloride in the portion of sample withdrawn at the specied time point (mg/mL) i
V = volume of Medium, 900 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn at each time point (mL)
Tolerances: See Table 5.
Time Point (i) | Time (h) | Amount Dissolved (%) |
1 | 1 | NMT 20 |
2 | 3 | 20–40 |
3 | 6 | 35–55 |
4 | 12 | 60–80 |
5 | 20 | NLT 80 |
The percentages of the labeled amount of alfuzosin hydrochloride dissolved at the times specied conform to Dissolution 〈711〉, Acceptance Table 2.
Test 6: If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 6.
Medium: 0.01 N hydrochloric acid; 900 mL
Apparatus 2: 100 rpm. Adjust the paddle height to 4.5 cm above the bottom of the vessel and use a 10# mesh basket as sinker. Times: 1, 6, 12, and 20 h
Buffer: 2.3 g/L of anhydrous dibasic sodium phosphate and 1.75 g/L of monobasic potassium phosphate
Mobile phase: Acetonitrile and Buffer (50:50)
Standard stock solution: 0.45 mg/mL of USP Alfuzosin Hydrochloride RS. Prepare by transferring a portion of USP Alfuzosin Hydrochloride RS to a suitable ask. Add 20% of the ask volume of water. Sonicate to dissolve, and dilute with Medium to volume. Standard solution: 0.011 mg/mL of USP Alfuzosin Hydrochloride RS in Medium from the Standard stock solution
Sample solution: Pass a portion of the solution under test through a suitable lter. Replace with the same volume of Medium. Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Column temperature: 30°
Flow rate: 1 mL/min
Injection volume: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Column eciency: NLT 3500 theoretical plates
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (Ci) of alfuzosin hydrochloride (C19H27N5O4 · HCl) in the sample withdrawn from the vessel at each time point (i):
Resulti = (rU /rS ) × CS
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Alfuzosin Hydrochloride RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of alfuzosin hydrochloride (C19H27N5O4 · HCl) dissolved at each time point (i):
Result1 = C1 × V × (1/L) × 100
Result2 = [(C2 × V) + (C1 × VS )] × (1/L) × 100
Result3 = {(C3 × V) + [(C2 + C1 ) × VS ]} × (1/L) × 100
Result4 = {(C4 × V) + [(C3 + C2 + C1 ) × VS ]} × (1/L) × 100
Ci = concentration of alfuzosin hydrochloride in the portion of sample withdrawn at the specied time point (mg/mL)
V = volume of Medium, 900 mL
L = label claim (mg/Tablet)
VS= volume of the Sample solution withdrawn at each time point and replaced with Medium (mL)
Tolerances: See Table 6.
Time Point (i) | Time (h) | Amount Dissolved (%) |
1 | 1 | NMT 30 |
2 | 6 | 45–65 |
3 | 12 | 70–90 |
4 | 20 | NLT 85 |
The percentages of the labeled amount of alfuzosin hydrochloride dissolved at the times specied conform to Dissolution 〈711〉, Acceptance Table 2.
Test 7: If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 7.
Medium: 0.01 N hydrochloric acid; 900 mL
Apparatus 2: 100 rpm with sinker; see Dissolution 〈711〉, Figure 2a.
Times: 1, 6, 12, and 20 h
Solution A: 500 g/L of sodium hydroxide
Solution B: 100 g/L of sodium hydroxide
Buffer: Add 5 mL of perchloric acid in 1000 mL of water. Adjust with Solution A to a pH of 2.5, then adjust with Solution B to a pH of 3.50 ± 0.05. Pass through a suitable lter of 0.45-µm pore size.
Mobile phase: Acetonitrile and Buffer (24:76)
Standard solution: 0.01 mg/mL of USP Alfuzosin Hydrochloride RS in Medium
Sample solution: Centrifuge or pass a portion of the solution under test through a suitable lter.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 245 nm
Column: 4.6-mm × 5-cm; 3-µm packing L7
Flow rate: 1.5 mL/min
Injection volume: 10 µL
Run time: NLT 1.5 times the retention time of alfuzosin
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 1.8
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration (Ci) of alfuzosin hydrochloride (C19H27N5O4· HCl) in the sample withdrawn from the vessel at each time
point (i):
Resulti = (rU /rS ) × CS
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Alfuzosin Hydrochloride RS in the Standard solution (mg/mL)
Calculate the percentage of the labeled amount of alfuzosin hydrochloride (C19H27N5O4 · HCl) dissolved at each time point (i): 19 27 5 4
Result1 = C1 × V × (1/L) × 100
Result2 = [(C2 × V) + (C1 × VS )] × (1/L) × 100
Result3 = {(C3 × V) + [(C2 + C1 ) × VS ]} × (1/L) × 100
Result4 = {(C4 × V) + [(C3 + C2 + C1 ) × VS ]} × (1/L) × 100
Ci = concentration of alfuzosin hydrochloride in the portion of sample withdrawn at the specied time point (mg/mL)
V = volume of Medium, 900 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn at each time point (mL)
Tolerances: See Table 7.
Time Point (i) | Time (h) | Amount Dissolved (%) |
1 | 1 | NMT 25 |
2 | 6 | 40–60 |
3 | 12 | 65–85 |
4 | 20 | NLT 85 |
The percentages of the labeled amount of alfuzosin hydrochloride dissolved at the times specied conform to Dissolution 〈711〉, Acceptance Table 2.
Uniformity of Dosage Units 〈905〉: Meet the requirements
5 IMPURITIES
Change to read:
5.1 Organic Impurities
Solution A, Mobile phase, Diluent, Sample solution, and Chromatographic system: Proceed as directed in the Assay. System suitability stock solution: 0.4 mg/mL of USP Alfuzosin System Suitability Mixture A RS in methanol System suitability solution: 0.03 mg/mL of USP Alfuzosin System Suitability Mixture A RS in Diluent from the System suitability stock solution Standard stock solution: 0.15 mg/mL of USP Alfuzosin Hydrochloride RS in methanol
Standard solution: 0.03 mg/mL of USP Alfuzosin Hydrochloride RS in Diluent from the Standard stock solution System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 1.0 between alfuzosin and the furamide analog; NLT 1.0 between deacylated alfuzosin and the N-formyl analog, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
5.2 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Tablets taken:
Result = (rU /rS ) × (CS /CU ) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of alfuzosin from the Standard (ERR 1-Nov-2021) solution
CS = concentration of USP Alfuzosin Hydrochloride RS in the Standard solution (mg/mL)
CU = nominal concentration of alfuzosin hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: See Table 8.
Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
Deacylated alfuzosina | 0.46 | 0.40 |
N-Formyl analogb | 0.50 | 0.30 |
Alfuzosin | 1.0 | — |
Furamide analogc | 1.18 | —d |
Any individual unspecied impurity | — | 0.20 |
Total impurities | — | 0.80 |
a N2-(3-Aminopropyl)-6,7-dimethoxy-N2-methylquinazoline-2,4-diamine.
b N-[3-[(4-Amino-6,7-dimethoxyquinazolin-2-yl)(methyl)amino]propyl]formamide.
c N-{3-[(4-Amino-6,7-dimethoxyquinazolin-2-yl)(methyl)amino]propyl}furan-2-carboxamide.
d Furamide analog, a component of USP Alfuzosin System Suitability Mixture A RS, is not a specied impurity.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Protect from light and moisture. Store at controlled room temperature.
Labeling: When more than one Dissolution test is given, the labeling states the Dissolution test used only if Test 1 is not used.
USP Reference Standards 〈11〉
USP Alfuzosin Hydrochloride RS
USP Alfuzosin System Suitability Mixture A RS
Furamide analog: N-{3-[(4-Amino-6,7-dimethoxyquinazolin-2-yl)(methyl)amino]propyl}furan-2-carboxamide.
C19H23N5O4 385.42
Deacylated alfuzosin: N2-(3-Aminopropyl)-6,7-dimethoxy-N2-methylquinazoline-2,4-diamine.
C14H21N5O2 291.35
N-Formyl analog: N-[3-[(4-Amino-6,7-dimethoxyquinazolin-2-yl)(methyl)amino]propyl]formamide.
C15H21N5O3 319.36


