Albuterol
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
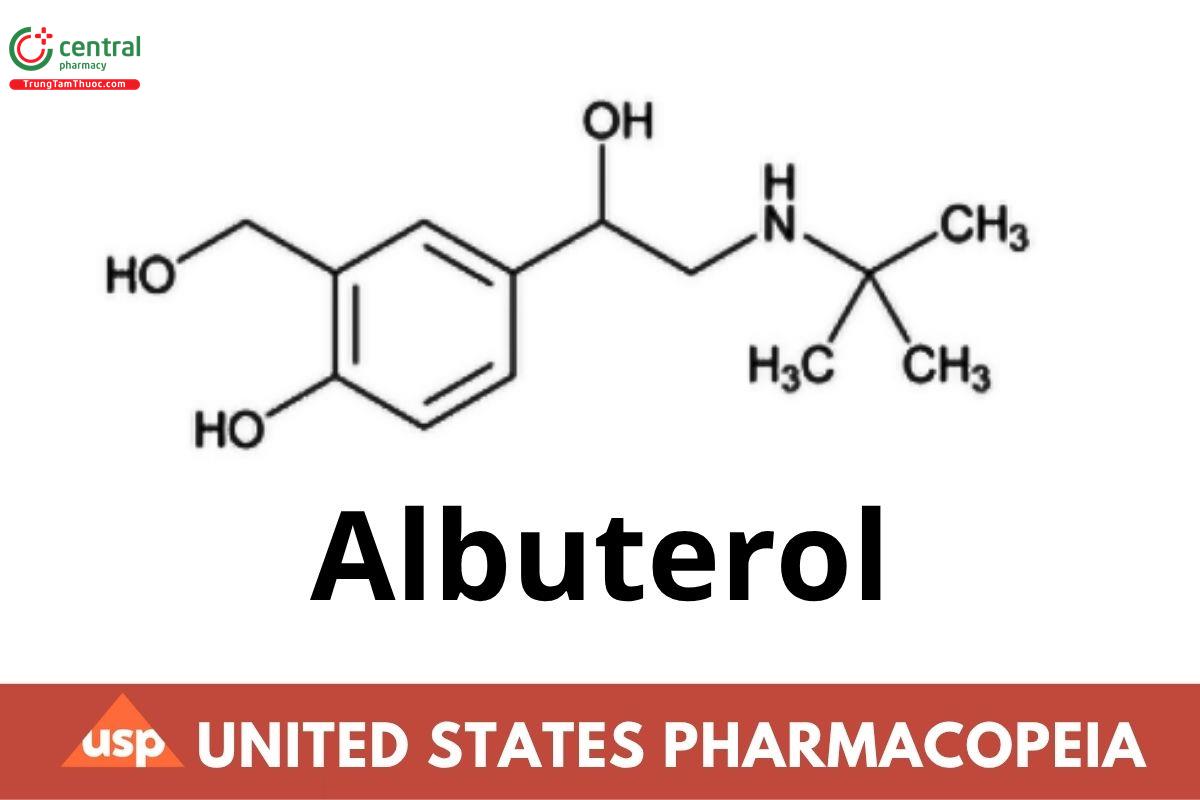
C13H21NO3 239.31
1,3-Benzenedimethanol, α1-[[(1,1-dimethylethyl)amino]methyl]-4-hydroxy-;
α1-[(tert-Butylamino)methyl]-4-hydroxy-m-xylene-α,α′-diol CAS RN®: 18559-94-9; UNII: QF8SVZ843E.
1 DEFINITION
Albuterol contains NLT 98.5% and NMT 101.0% of albuterol (C13H21NO3), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K(CN 1-May-2020)
Change to read:
B. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U(CN 1-May-2020)
Sample solution: 80 µg/mL in 0.1 N hydrochloric acid
Acceptance criteria: Meets the requirements
3 ASSAY
Procedure
Sample solution: 8 mg/mL of Albuterol in glacial acetic acid
Analysis: To 50 mL of the Sample solution add 2 drops of crystal violet TS, and titrate with 0.1 N perchloric acid VS. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 23.93 mg of C13H21NO3.
Acceptance criteria: 98.5%–101.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Organic Impurities
Standard solution: 0.10 mg/mL of USP Albuterol RS in methanol
Sample solution: 20 mg/mL of Albuterol in methanol
4.1 Chromatographic system
(See Chromatography 〈621〉, Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel
Application volume: 10 µL
Developing solvent system: Methyl isobutyl ketone, isopropyl alcohol, ethyl acetate, ammonium hydroxide, and water (50:45:35:3:18) Visualization: Iodine vapor
4.2 Analysis
Samples: Standard solution and Sample solution
Proceed as directed in the chapter, applying aliquots of the Standard solution and the Sample solution. Develop in the Developing solvent system until the solvent front has moved three-fourths the length of the plate. Remove the plate from the developing chamber, air-dry, and expose it to iodine vapor.
Acceptance criteria: Any spot, other than the principal spot, obtained from the Sample solution is not greater in size and intensity than the spot produced by the Standard solution (0.5%), and the sum of the impurities is not greater than 2.0%.
5 SPECIFIC TESTS
Water Determination, Method I〈921〉: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed, light-resistant containers.
USP Reference Standards 〈11〉
USP Albuterol RS


