Acetohydroxamic Acid
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C2H5NO2 75.07
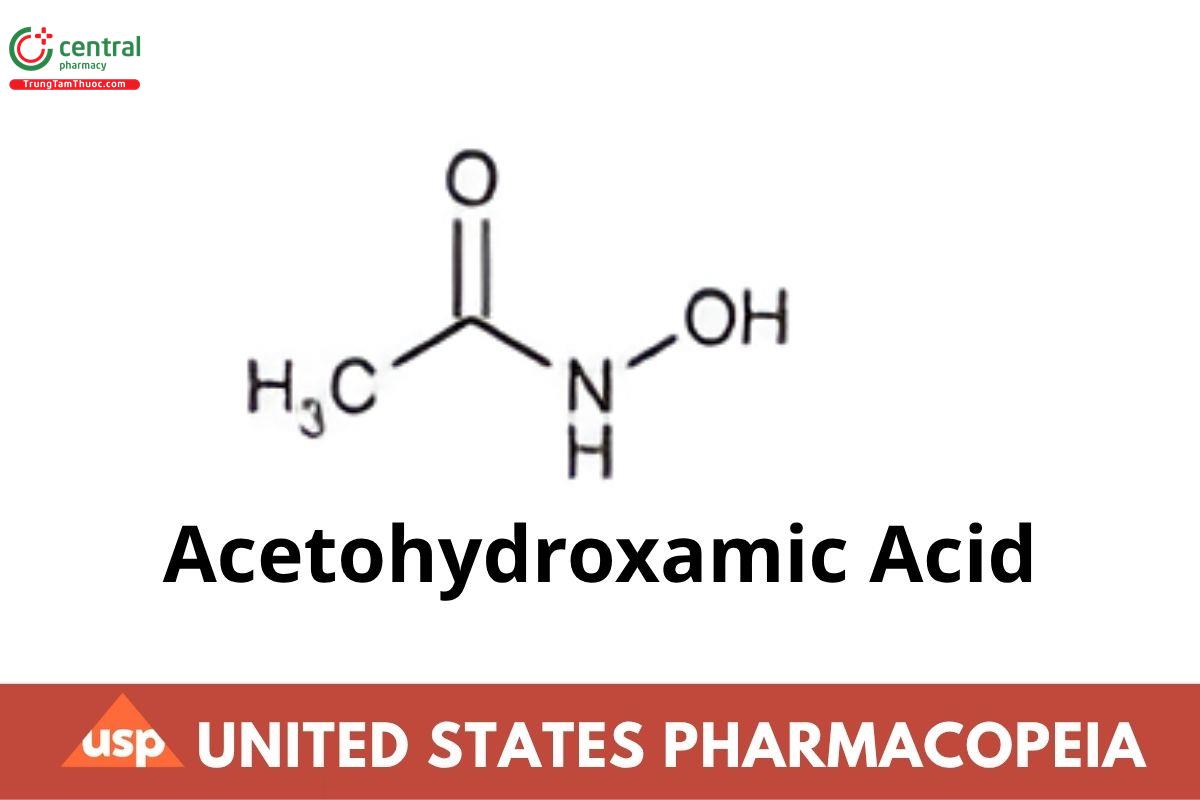
N-Acetyl hydroxyacetamide;
Acetohydroxamic acid CAS RN®: 546-88-3; UNII: 4RZ82L2GY5.
1 DEFINITION
Acetohydroxamic Acid, dried over phosphorus pentoxide for 16 h, contains NLT 98.0% and NMT 101.0% of acetohydroxamic acid (C2H5NO2).
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B.
Sample solution: 20 mg/mL in water
Analysis: To 10 mL of the Sample solution add 2 drops of potassium permanganate TS.
Acceptance criteria: The pink color of the permanganate disappears.
3 ASSAY
Procedure
Ferric chloride solution: 20 mg/mL of ferric chloride in 0.1 N hydrochloric acid
Standard solution: 500 μg/mL of USP Acetohydroxamic Acid RS in 0.1 N hydrochloric acid
Sample solution: 500 μg/mL of Acetohydroxamic Acid, previously dried, in 0.1 N hydrochloric acid
Blank: 0.1 N hydrochloric acid
Analysis
Samples: Standard solutions, Sample solution, and Blank
Transfer 10.0 mL each of the Standard solution, Sample solution, and Blank to separate 100-mL volumetric flasks. To each flask add 50 mL of 0.1 N hydrochloric acid and 10.0 mL of Ferric chloride solution, and dilute with 0.1 N hydrochloric acid to volume. Without delay, concomitantly determine the absorbances of the solutions at the wavelength of maximum absorbance at about 502 nm using the Blank to set the instrument.
Calculate the percentage of acetohydroxamic acid (C2H5NO2) in the portion of Acetohydroxamic Acid taken:
Result = (Au/As) × (Cs/Cu) × 100
Au = absorbance of the Sample solution
As = absorbance of the Standard solution
Cs = concentration of USP Acetohydroxamic Acid RS in the Standard solution (μg/mL)
Cu = concentration of Acetohydroxamic Acid in the Sample solution (μg/mL)
Acceptance criteria: 98.0%–101.0% on the previously dried basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Limit of Hydroxylamine
Buffer: 1.36 g/L of monobasic potassium phosphate in water, adjusted with 1 M potassium hydroxide to a pH of 7.4
Solution A: 1 mg/mL of pyridoxal 5-phosphate monohydrate in Buffer, prepared in a low-actinic flask fresh before use
Standard stock solution: 2.0 mg/mL of hydroxylamine hydrochloride in water
Standard solutions: Transfer 5.0, 10.0, and 15.0 mL of the Standard stock solution to separate 100-mL volumetric flasks, and dilute with water to volume.
Sample solution: Transfer 1500 mg of Acetohydroxamic Acid, previously dried, to a 100-mL beaker, and dissolve in a sufficient amount of water to cover the electrode of a calibrated pH meter (about 60 mL). While stirring, adjust with 0.05 M potassium hydroxide to a pH of 7.4.
Transfer the contents of the beaker, with the aid of small portions of water, to a 100-mL volumetric flask, and dilute with water to volume.
Blank: Water
Analysis
Samples: Standard solutions, Sample solution, and Blank
Transfer 2.0 mL of each Standard solution, the Sample solution, and Blank into separate 100-mL volumetric flasks. To each flask add 4.0mL of Solution A. After 8 min, accurately timed, dilute the contents of each flask with Buffer to volume.
Immediately determine the fluorescence intensities of the solutions from the Standard solutions and the Sample solution in a fluorometer at an excitation wavelength of 350 nm and an emission wavelength of 450 nm, setting the instrument to zero with the Blank. Determine the best-fit straight line from the fluorescence intensities of the three Standard solutions versus the hydroxylamine hydrochloride concentrations, in μg/mL. From the best-fit straight line, determine the concentration, in μg/mL, of hydroxylamine hydrochloride in the Sample solution.
Calculate the percentage of hydroxylamine in the portion of Acetohydroxamic Acid taken:
Result = (Cu/C) × (Mr1/Mr2) × 100
Cu= concentration of hydroxylamine hydrochloride in the Sample solution (mg/mL)
C = concentration of Acetohydroxamic Acid in the Sample solution (mg/mL)
Mr1 = molecular weight of hydroxylamine, 33.03
Mr2 = molecular weight of hydroxylamine hydrochloride, 69.50
Acceptance criteria: NMT 0.5%
5 SPECIFIC TESTS
Loss on Drying 〈731〉
Analysis: Dry a sample over phosphorus pentoxide for 16 h.
Acceptance criteria: NMT 1.0%
Completeness of Solution 〈641〉: A 1.0-g portion dissolves in 10 mL of water to yield a clear solution.•
Color of Solution
Sample solution: 200 mg/mL in water
Blank: Water
Instrumental conditions
Mode: UV-Vis
Analytical wavelength: Between 400 and 750 nm
Cell: 1 cm
Acceptance criteria: The absorbance is NMT 0.050.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers, and store in a cool, dry place.
USP Reference Standards 〈11〉
USP Acetohydroxamic Acid RS


